Serialization and aggregation in the pharmaceutical industry: why outsource these services?
The pharmaceutical industry faces highly stringent and restrictive requirements. This is due to the critical importance of ensuring the quality of medicinal products, which often directly affects the health and lives of patients. In recent years, demands for safety and transparency in the pharmaceutical sector have significantly increased. The introduction of strict regulations, such as the so-called “Anti-Counterfeiting Directive” (Falsified Medicines Directive, FMD), has compelled manufacturers to adopt advanced technological solutions for product monitoring and traceability. In this context, serialization and aggregation processes have become pivotal, playing a fundamental role in protecting medicines from counterfeiting and ensuring the transparency of supply chains.
On February 9, 2019, compliance with the requirements of the EU Delegated Regulation (EU) 2016/161, derived from Directive 2011/62/EU of June 8, 2011, officially began. This regulation, commonly referred to as the Anti-Counterfeiting Directive, aims to prevent falsified medicinal products from entering the legitimate supply chain.
What is serialization?
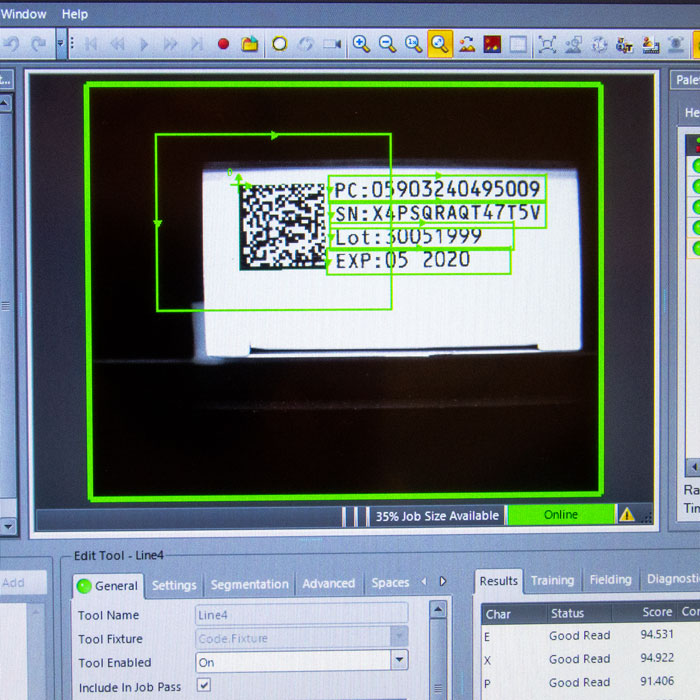
Serialization involves assigning a unique code to each unit of a medicinal product, enabling precise tracking of its journey from production to the patient. Additionally, this process requires printing the data in a 2D Data Matrix code, which must include the product code, batch number, expiration date, and a unique serial number generated through a computerized algorithm. This algorithm must not repeat for at least five years after market introduction.
In the pharmaceutical industry, serialization is crucial in combating counterfeit drugs, which pose a significant public health risk. It allows real-time monitoring of each product unit, enabling swift action in cases of suspected falsification or quality issues. Serialization has become mandatory across Europe, ensuring the safety and authenticity of medicinal products.
From February 9, 2019, drug manufacturers are required to include two safety features on packaging:
- A unique identifier (UI) in the form of a 2D code.
- An anti-tampering device (ATD) to verify that packaging has not been physically compromised.
The 2D code must contain:
- Batch or lot number (Batch, Lot).
- Unique serial number for the packaging (SN, NS).
- Expiration date (EXP).
- Product code (GTIN, NTIN, PC).
Serialization ensures the identification and verification of each product package throughout its market lifecycle and even beyond, for returns and disposal after expiration.
Serialization will soon become obligatory across the entire European Union. Italy, Belgium, and Greece—the final three countries—will comply with these regulations starting in February 2025.
What is aggregation?
Aggregation is closely tied to serialization. It involves linking individual units of pharmaceutical products to larger packaging groups, such as cartons or pallets. This allows the tracking of entire product batches at various levels, from single packages to larger collective units, without the need to scan each item individually at every stage of the supply chain.
Aggregation complements serialization by enhancing supply chain efficiency and improving logistics management. It also facilitates quality control, enabling immediate tracking of entire product batches in case of irregularities or counterfeiting concerns.
Several countries have already introduced mandatory aggregation requirements. Turkey, Egypt, and India have implemented such measures, and since 2020, aggregation has been mandatory in the Commonwealth of Independent States (CIS) and Pakistan. Countries like Japan, Brazil, South Africa, and the United States have followed suit, making aggregation a global standard. Without aggregation, exporting products to these countries may be prohibited.
Challenges of serialization and aggregation for pharmaceutical companies
Serialization and aggregation are mandatory in many countries, requiring pharmaceutical companies to comply to distribute their products. These processes present several challenges, including:
- Investment in equipment: Serialization requires purchasing specialized machinery, training personnel, and maintaining systems.
- Data management: Handling vast amounts of data, including serial numbers across global supply chains, demands advanced IT infrastructure.
- Operational costs: High costs for equipment, software updates, and training increase operational expenses.
Benefits of outsourcing serialization and aggregation
Outsourcing these processes offers a cost-effective solution to the challenges of meeting regulatory and technological demands. External service providers specialize in serialization and aggregation, offering:
- Cost optimization: Reduced expenses on infrastructure, personnel, and maintenance.
- Focus on core business: Companies can prioritize R&D and market expansion instead of managing packaging operations.
- Regulatory compliance: Expert knowledge ensures adherence to strict pharmaceutical regulations.
- Scalability: External providers adapt services to fluctuating market demands.
- Professional expertise: Access to state-of-the-art technologies and best practices enhances competitiveness.
Summary
The growing importance of serialization and aggregation in the pharmaceutical industry necessitates that companies adapt to evolving regulations. These processes are essential for ensuring product safety and supply chain control but come with significant costs and complexities. Outsourcing these operations provides a strategic advantage, enabling companies to leverage modern technologies and expertise while focusing on their core business.
Partnering with specialists like Prespack ensures streamlined processes, regulatory compliance, and competitive edge. Let’s discuss how we can help grow your business.