Trends in 2023 for the pharmaceutical industry and Prespack’s plans for further development
The pharmaceutical industry is one of many that in this year will have to face changes resulting from new legal regulations (e.g. for medical devices) or market factors. So what transformations await the pharmaceutical industry in 2023? Which trends will affect this sector? What are the challenges facing our company and what awaits us in the coming months? We decided to share our predictions on this issues and we want to present what goals we set for ourselves in 2023.
Outsourcing of services in the pharmaceutical industry
Outsourcing part of the production processes to an external company has been common in the pharmaceutical industry for many years. Outsourcing, however, is expanding to new areas every year and this is a trend that will develop. Pharmaceutical companies very often outsource contract packaging, thanks to which they reduce costs in areas such as logistics, storage, labeling as well as serialization and aggregation. Such action brings many benefits, which are increasingly appreciated by manufacturers of medicines and medical devices. We think about:
- lowering operating costs,
- reducing the costs of employing specialists,
- transfer of batch certification to the packaging company.
Outsourcing is a good solution for companies that focus on development and want to run their projects in an effective way. For many years, we have been providing specialized contract packaging services as well as serialization and aggregation of medicinal products, products containing psychotropic substances and other products requiring storage in the cold chain. We know how to optimize processes so that the ordered services are always of the highest quality.
Digitization of processes
This is another important subject for this industry. Optimization of manufacturing and packaging processes is possible through the implementation of electronic recording of production processes. This is due to the need to increase its efficiency and quality. Knowledge about the current process and the possibility of its thorough analysis in real time became very important. The implementation of these goals is possible thanks to the creation and implementation of an appropriate computerized system. At Prespack, we know that in order to adapt to the market situation and to meet the client's requirements, as a company specializing in contract packaging of medicinal products and products containing psychotropic substances, we had to develop a system that would automate the collection and verification of data on the progress of production. This is a challenge facing the entire industry to better control the efficiency of the services provided
Changes in the law – UDI
The revolution in the medical devices market is continuing. In 2017, Regulation 2017/745 of the European Parliament and of the Council on medical devices (MDR-Medical Device Regulation) was adopted. The regulation was supposed to apply from 2020, but in the face of the coronavirus pandemic, its application was postponed. The main objective of the MDR is to introduce a uniform Community regulatory framework for the market of medical devices in the European Union. All devices are subject to UDI marking, except for those made to order and those in the clinical trials phase.
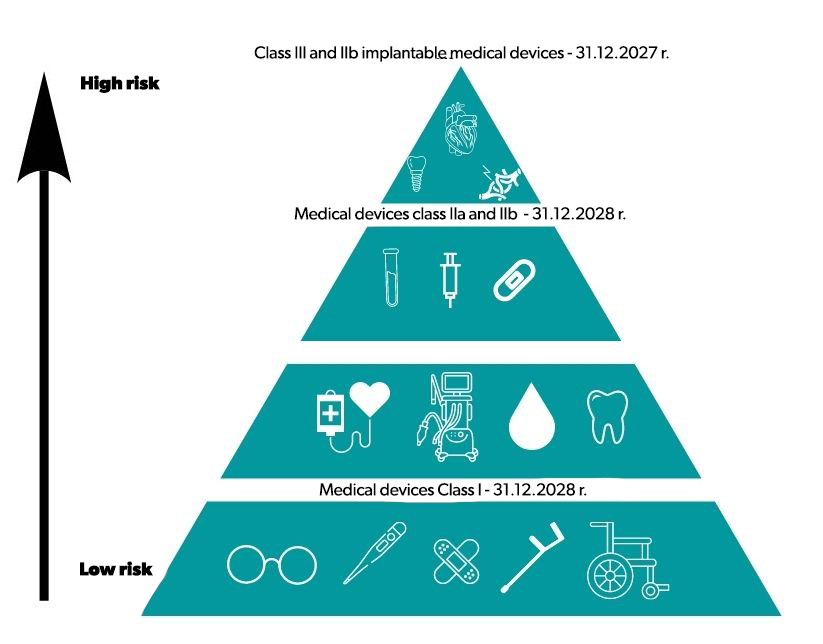
However, the legislator provided for the introduction of transitional periods for the new regulations in order to limit the risk of shortages. On March 20, 2023, a regulation amending the MDR was published in the Official Journal of the European Union regarding the extension of the transitional periods for medical devices with certificates. The new dates depend on the risk class of medical devices and will ensure that patients have access to these devices at all times. It will also allow medical devices placed on the market in accordance with the applicable legal framework and still available (i.e. without a sell-out date) to remain on the market.
What does the new regulation change? Certificates issued by notified bodies valid on May 26, 2021, which have not been withdrawn after this date, remain valid after the end of the period indicated in the certificate until December 31, 2027 for implantable class III and IIb devices and until December 31, 2028. for class IIb, IIa and class Is and Im devices.
However, class I products according to MDD, which according to MDR should be classified as a higher class, may be placed on the market until December 31, 2028, however, under certain specific conditions.
In addition, the end date (sell-off clause) was abandoned, thanks to which medical devices placed on the market before the entry into force of the MDR may still be made available on the market and put into service without an end date.
At Prespack, we constantly monitor changes in legal regulations. We make every effort to help our current and future clients implement them properly, in accordance with the legal requirements of a given country.
Security guarantee - pharmaceutical serialization and aggregation service
Security and full traceability are very important factors. Pharmaceutical serialization and aggregation are two complementary processes that facilitate the achievement of the above goals. Many companies that have completed the process of implementing serialization of medicinal products envisage aggregation as the next step. It offers new possibilities in the process of tracking medicines in the supply chain. Thanks to the use of these solutions, pharmaceutical companies achieve significant savings. It is also inherent in the modernization of the production process. Aggregation allows for radical time savings. Thanks to it, we eliminate the need to open each batch or collective packaging in order to find and scan a single product. All data is easily accessible in the supply chain. This makes it easy to track the products in each transaction. This has a positive effect on the efficiency of the distribution process, and consequently on the safety and reliability of the processes. By analyzing trends and changes resulting from the needs of our clients, our offer includes both serialization and pharmaceutical aggregation services. In this way, we want to provide our Partners with additional opportunities to secure their products.
Landmark investment in Prespack
In 2022 we’ve been continued the construction of a new Prespack production site. It was a milestone and in November we officially signed the notarial deed and handed over the building for use. Finishing works are in progress and, hoping that everything will go according to the work schedule, we plan to start contract production in the new location in the fourth quarter of this year. It will be a big challenge, but the new production site will increase our production capacity and allow us to provide services at an even higher level. We have a lot of work ahead of us and new challenges, but we look to the future with optimism, hoping for further development of our business.