Serialization enters the Italian market. What challenges await companies due to these changes?
February 9, 2025, is an important date for the Italian pharmaceutical market. On that day, the last three EU member states will implement the European pharmaceutical serialization system.
The Directive on Falsified Medicines provides a legal framework introduced by the European Commission to enhance public health protection within the European Union. The Falsified Medicines Directive 2011/62/EU (EU FMD) came into effect on February 9, 2019. However, Italy, Belgium and Greece were exempted for a six-year period because these countries already had existing verification and traceability systems for medicinal products. As mentioned, while The Falsified Medicines Directive was introduced in February 2019, these regulations were postponed in Italy due to the pre-existing Bollino system. As we approach 2025, the transitional period is ending, making it crucial for companies to adequately prepare for regulatory changes.
Bollino system
In Italy, the authenticity of medicinal products and the identification of individual packages are guaranteed by the Bollino system. To be able to sell medicinal products in Italy, each company must manage the Bollino process, purchasing labels and submitting data to the database of the Italian Ministry of Health. Bollino is a single label that is applied to each package and is uniquely identified. You must follow a procedure to register your products as a legal entity, and the entire system is supervised by the Ministry of Health. Each Bollino label includes a serial number, but this must be purchased and assigned to a batch. The label is applied to the packaging, and then the Ministry of Health must be notified about the use of the list for a given batch or removal from circulation.
The Bollino system is used to control government spending on public health and monitor the entire supply chain. Every participant in the production and distribution of medicines, including pharmacies, is tracked and registered. So in Italy, currently every participant in the distribution chain is identified and monitored.
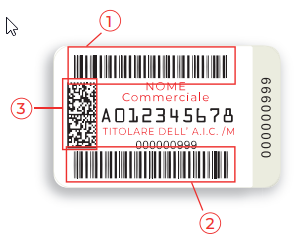
Unlike the rest of the EU countries, packaging labels (the so-called Bollino labels) and serial numbers are issued by the state. Another unique feature is that tracking data is linked to health information. For example, information on medicines safety and medicines reimbursement. The manufacturer buys the label from the Italian Printing Office (IPZS) and sticks it on the medicine packaging. It places important drug data in the form of letters, two barcodes and a matrix code.
The top code (1) contains the AIC code, an authorization number with nine digits that is issued by the Italian Medicines Agency (A.I.F.A.). The serial number (UID) for unique identification of the package is located in the lower barcode (2). It is also required from the Italian Printing Authority (IPZS). Finally, matrix code (3) summarizes both code information, AIC code and UID.
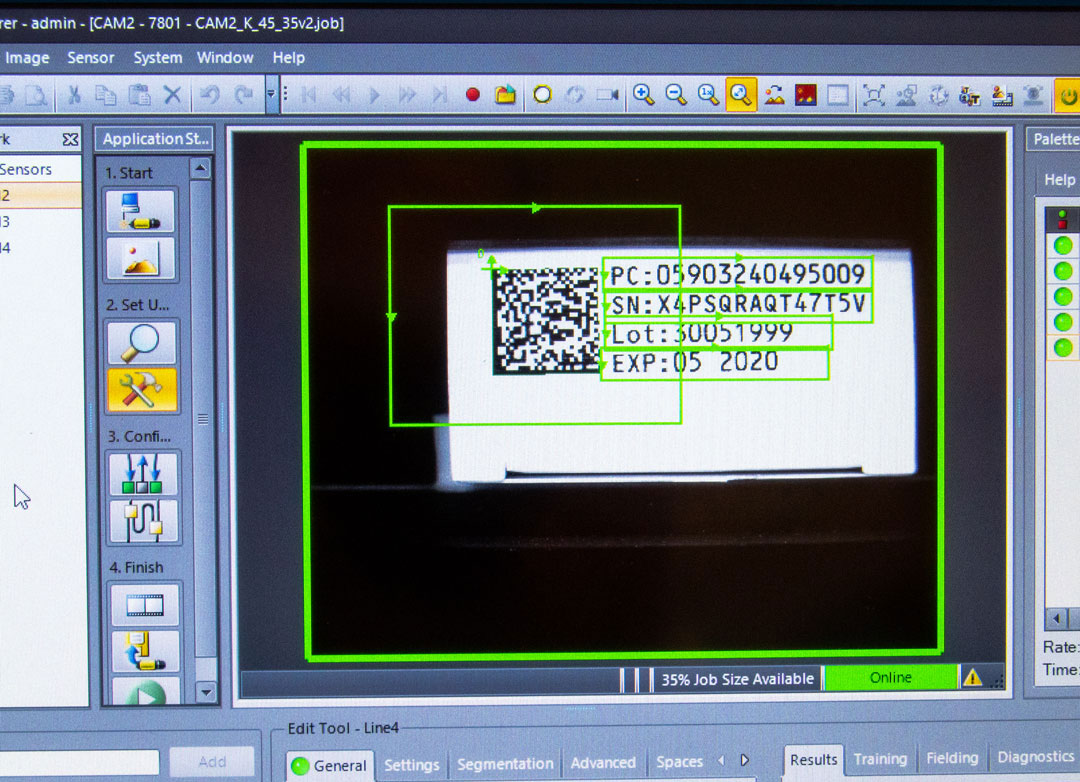
A central database allows relevant authorities to track every pharmaceutical packaging in the supply chain. The database is part of the Italian National Health Information System (NHIS). This makes it possible to monitor all information regarding an individual medicinal product. Each incident, whether a suspected counterfeit drug or an adverse reaction, can therefore be directly tracked for a specific drug. This makes immediate countermeasures possible.
What is the Falsified Medicines Directive 2011/62/EU (EU FMD) and why was it introduced?
One of the requirements for improving the quality of medicines is their traceability. This is to protect the health and life of patients against falsified medicines. Directive 2011/62/EU of the European Parliament and of the Council of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use - as regards the prevention of the introduction of falsified medicinal products into the legal distribution chain, hereinafter referred to as the Directive The Falsified Medicines Directive (FMD), was adopted to protect the European distribution chain against the introduction of falsified medicinal products. It introduced the obligation to place two types of security features on prescription medicines: a unique identifier (UI) in the form of a two-dimensional code and elements indicating the opening of the package (ATD).

Serialization provides a unique number assigned to each unit of a medicinal product. The number provided can be used to traceability and authenticate products in the distribution chain, allowing the identification of any counterfeit medicines in circulation. There are several essential elements that should be distinguished in the serial number:
- batch number of the medicinal product,
- serial number of the packaging,
- expiration date,
- product code.
The serial number must be unique and must not be repeated for at least five years after its introduction to the market. The secured medicines are checked in the pharmacy by reading the code. We receive confirmation of its authenticity from the integrated computer system, which allows us to ensure patient safety. If the process shows non-compliance, the computer system generates a warning, the medicinal product is withdrawn and then checked. The mechanism for serialization and verification of medicinal products applies only to prescription drugs and a few over-the-counter medicines.
Combined with Track and Trace technology, serialization makes it easier to track your product throughout the supply chain. Global Standards one (GS1) is a not-for-profit organization that develops global standards for identifying goods and services. GS1 standards are used to identify pharmaceutical products in 60 countries around the world. Most of the world requires product serialization according to the GS1 2D Data Matrix code standard. The 2D Data Matrix Code contains various data which typically includes a company ID, global trade number, country product code, expiration date and additional fields. As the serialization process varies depending on national laws and regulations, several differences may be observed.

Companies that had previously produced medicinal products exclusively for the Italian market only used the Bollino system. However, if the production was intended for European markets, security in the form of a Unique Identifier and ATD became obligatory.
How are these systems different?
|
Bollino system |
Serialization |
|
Bollino labels and serial numbers are issued by the state. |
You create a unique identifier yourself. |
|
The pharmaceutical manufacturer buys the label from the Italian Printing Office (IPZS) and sticks it on the medicine packaging. It places important drug data in the form of letters, two barcodes and a 1D matrix code. |
Data presented using a 2D Data Matrix code compliant with the GS1 standard and readable text. In the Unique Identifier you need to enter several essential elements: batch number of the medicinal product, serial number of the packaging, expiry date, product code. |
|
The AIC code, an authorization number with nine digits, is issued by the Italian Medicines Agency (A.I.F.A.). The serial number (UID) to uniquely identify the package is located in the bottom barcode. It is also required from the Italian Printing Authority (IPZS). |
Packaging units are marked with a global trade number (GTIN), batch number, expiration date of the medicinal product and a randomly generated serial number. The latter can be generated by producers themselves. GTIN, on the other hand, is issued once by GS1 and is valid worldwide. |
|
The database is part of the Italian National Health Information System (NHIS). |
The data is sent to the European central (EUHUB) – global approach. |
What challenges do pharmaceutical companies face with the entry into force of serialization regulations in Italy?
First of all, awareness of the upcoming changes in the security of medicines on the Italian market from February 9, 2025. The companies will have to review their products and change their artwork to prepare their medicinal products for release to the market due to new regulations. The whole process, depending on the size of the company, may take a really long time, so it is not worth leaving it until the last minute. Unfortunately, some companies have still not started work in connection with the entry into force of the new serialization regulations.
Another challenge is to meet the financial requirements related to the purchase and launch of appropriate hardware and software, validation of its connection and its maintenance, annual license fees and daily maintenance. When switching from the Italian Bollino system to The Falsified Medicines Directive, many changes will have to be introduced, which may prove costly.
When should companies start preparing to meet EU-FMD requirements and what are the risks incurred by companies that decide to postpone these plans?
Many companies have already started preparations, but there is still a group of entities that have not addressed this topic. As we have already mentioned, depending on the size of the company, the process can be time-consuming and expensive to adapt to upcoming requirements. Serialization is a complex project that involves the work of many internal departments and different vendors. The earlier preparations start, the better to avoid future problems and delays in the availability of medicines on the Italian market.
How can Prespack help you with implementation?
The transition from the Italian 1D Bollino code system to the EU 2D Data Matrix code is not just a change of visual aspects. It is a matter of implementing global compliance standards, data exchange in the supply chain and technical preparation. We know this all may sound very complicated. Implementing serialization in your company can also be very costly. But we can help you with this.
Prespack has been supporting pharmaceutical companies in implementing serialization projects for many years. We know the challenges that small and medium-sized enterprises especially have to face in this area. We have completed many outsourcing projects for packaging products requiring serialization. Our services are not limited to one-time compliance with regulations.
Thanks to our many years of experience, you can gain a partner who develops and is up to date with regulations regarding the pharmaceutical market in Europe. We provide optimal support for your business processes.
Ask about cooperation details. We will help you implement solutions in accordance with the regulations coming into force in Italy, so that you can take care of your "core business".
Prespack. Your contract packaging partner.